Abstract
Introduction:
Chronic myelomonocytic leukemia (CMML) is a chronic myeloid malignancy associated with monocytosis, autoimmunity (~30%) & an inherent risk for leukemic transformation. Bone marrow (BM) dendritic cell (DC) populations occur in ~30% of patients, with a poorly defined biological & prognostic role. The malignant immune microenvironment is regulated by indoleamine 2,3-dioxygenase-1 (IDO-1) expressing DCs, which modulate regulatory T (Treg) cells & block their conversion into proinflammatory T helper (Th17)-like cells. IDO-1 is a known immune checkpoint & functions by catabolizing tryptophan, an amino acid essential for T cell function. We hypothesized that distinct IDO-1 expressing DC populations in CMML modulate Tregs & contribute towards immune tolerance & aggressive disease biology.
Methods:
Primary diagnostic CMML peripheral blood mononuclear cells (PBMC) & BM biopsy specimens were obtained after Mayo Clinic IRB approval. A DC population was defined on H&E stained biopsy sections as focal collections (>10) of cells with characteristic elongated nuclei & cytoplasmic extensions. Transcriptomic & protein expression studies assessing IDO-1 expression were done by previously described methods. In addition, IHC expression of PD-1, PD-L1 & CTLA-4 was also done. IDO-1 promoter methylation studies with DIP-seq were performed. The impact on immune tolerance was assessed using mass cytometry (CyTOF).
Results:
Cohort: Twenty eight patients with CMML were included in the study, median age 70 (range: 51-80) years; 71% males. Eleven (39%) patients had coexisting autoimmune conditions. Of these, 8 (73%) had detectable DC populations either at diagnosis, or during the course of their disease. At a median follow-up of 46 (95% CI 27, 84) months, there were 14 (50%) deaths & 9 (32%) leukemic transformations.
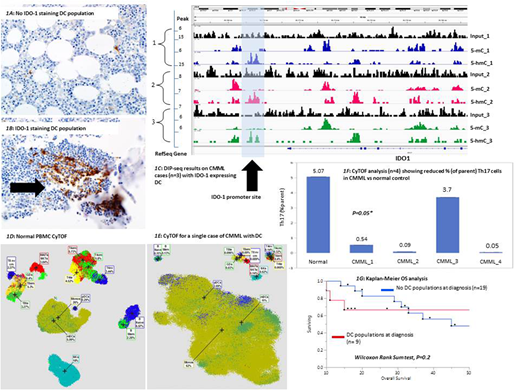
IHC results: Nine (32%) patients were identified to have a DC population at CMML diagnosis. CD123 & TCL1 staining was performed in 5 (56%) patients, with 3 being positive for both, & 2 positive for CD123 only (additional IHC studies ongoing). IDO1 expression by IHC was documented in all 9 (100%) cases (Fig 1A & 1B), while rare populations of PD-1, PD-L1 & CTLA-4 lymphocytes were also seen in all cases. Due to the low DC burdens (median cellularity ≤ 5%) & uniform staining intensity, IHC-based grading was not done. Samples at serial time-points, post-HMA therapy & at the time of blast transformation, were available in 5 & 3 patients respectively. Among the patients who did not have DC populations at diagnosis, 5 (42%) developed them post-HMA therapy, while 3 (50%) developed them at the time of LT. The development of DC populations was associated with loss of response to HMA (50%) & disease progression (50%).
Transcriptomic analysis: RNA expression data was available on 7 (25%) patients, of whom only 1 (14%) had DC populations at diagnosis. The IDO-1 RPKM value in the former was higher than the mean pooled value in the latter group (330 versus 74, p=0.05).
Methylation studies: DIP-seq was performed on 12 (43%) cases from the primary IHC cohort. Qualitative analysis of IDO-1 promoter hypomethylation was conducted & confirmed in all 9 (100%) cases with 5-mC & 5-hmC marks compared to input as displayed in figure 1C.
Immune profiling: CyTOF was performed on 4 CMML samples (3 with IDO-1 expressing DC populations at diagnosis) from the primary IHC cohort & compared to a normal PBMC control. Results confirmed an increase in DC populations (fig 1D& 1E), & reduced % of Th17-like T cells in CMML samples compared to control (1.1 versus 5.07, p=0.05, fig 1F).
Clinical correlates & survival analysis: With the exception that CMML patients with DC populations had a higher frequency of NRAS (P=0.007) mutations, the two groups were comparable for cytogenetic & molecular abnormalities. The median OS for the cohort was 45 (95% CI 29, 84) months. CMML patients with IDO-1 expressing DC populations at diagnosis had a shorter median OS, in comparison to those without (median OS 30 vs 45, p=0.03, Kaplan-Meier analysis in fig 1G).
Conclusions:
In conclusion, we demonstrate that DC populations are seen in ~30% of patients with CMML with a uniform expression of IDO-1 & limited expression of PD-1, PD-L1 & CTLA-4. CMML patients with BM DC populations have a higher frequency of NRAS mutations & DC IDO-1 expression is associated with tumor induced immune tolerance. Additional IHC, genomic & preclinical studies with IDO-1 inhibitors are ongoing.
Al-Kali:Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.